Challenge
Your challenge in this lab is to determine the exact percentage of sodium bicarbonate in a mixture of sodium bicarbonate and sodium carbonate using heat decomposition. By carefully measuring the mass loss due to the release of water and carbon dioxide gas, you will apply your knowledge of stoichiometry to calculate the composition of the mixture.
Prelab Questions
- What is the main difference between a mixture and a compound, and how can mixtures be separated into their components?
- Give one example of a physical separation method (e.g., filtration, distillation, chromatography, evaporation) and explain how it works. How is heating sodium bicarbonate similar to these methods of separation?
- Write the balanced chemical equation for the thermal decomposition of sodium bicarbonate. Which products are gases, and how does this explain the mass loss observed during the experiment?
- According to the balanced equation, what is the mole ratio of sodium bicarbonate to carbon dioxide produced? How will this relationship help you calculate the original amount of NaHCO₃ in the mixture?
- Why is sodium carbonate (Na₂CO₃) considered stable under the heating conditions of this lab, and why is this stability essential for determining the percent composition of NaHCO₃ in the mixture?
Background
Mixtures consist of two or more substances physically combined, where each substance retains its chemical identity and properties. Unlike chemical compounds, mixtures can be separated into their individual components through physical methods that exploit differences in physical properties such as particle size, density, solubility, boiling point, or magnetic susceptibility.
Common physical separation techniques include filtration, distillation, chromatography, and evaporation. For example, filtration is used to separate solids from liquids in heterogeneous mixtures, while distillation separates components based on differences in boiling points. Chromatography exploits differences in solubility and interaction with a stationary phase to separate components in a mixture, and evaporation can be used to remove a solvent, leaving the solute behind.
In the context of this lab, heat decomposition serves as a physical separation method. When heated, sodium bicarbonate decomposes into sodium carbonate, water vapor, and carbon dioxide gas, which are released as the solid mass decreases. Sodium carbonate remains stable under these conditions, so only the mass of sodium bicarbonate will change.
This method highlights a critical aspect of chemistry: using physical processes to isolate and quantify substances in a mixture. Understanding and applying separation techniques are essential skills in analytical chemistry, as they enable chemists to identify, purify, and measure components within complex mixtures, whether in industrial applications, environmental testing, or laboratory research.
In this lab, students will explore the composition of mixtures by determining the percentage of sodium bicarbonate (NaHCO3) in a mixture containing both sodium bicarbonate and sodium carbonate (Na2CO3). This analysis utilizes heat decomposition, a process where sodium bicarbonate decomposes when heated to form sodium carbonate, water, and carbon dioxide gas according to the following reaction:
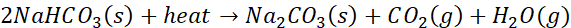
The thermal decomposition of sodium bicarbonate is significant because it allows for the separation of components within the mixture based on their differing chemical properties. Sodium carbonate, in contrast, does not decompose under the conditions used in this lab, making it possible to quantify the percentage of sodium bicarbonate in the mixture.
Upon heating, the mixture loses mass due to the production of CO2(g) and H2O(g). The mole ratio of H2O and CO2 is 1:1, meaning the moles of CO2 produced is equal to the moles of H2O produced. We will represent this by assigning the variable x to both moles of CO2 and moles of H2O. Using x for moles of CO2 and H2O, as well as their molar masses, we can solve for x:

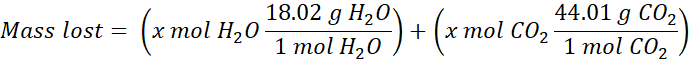
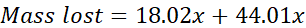
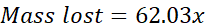
Once the moles of H2O or CO2 (x) is found, stoichiometry can be used to determine the grams of NaHCO3 that reacted in the mixture. Using the grams of NaHCO3 and the total mass of the mixture, you can determine the percent NaHCO3 that was present in the mixture. This is the goal of the experiment.
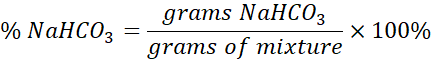
Post Lab Instructions
Lab Notebook – Complete in Notebook
- Ensure all sections from the Lab Report Guidelines and Expectations are included in the lab notebook. Label each section clearly.
- Lab Notebook Set Up: experiment number, name of the experiment, and partners.
- Purpose or Question: Describe what the experiment is testing or exploring and why it matters in a couple of sentences.
- Methods: Briefly describe what you did so someone else could repeat it.
- Data and Observations: Include both quantitative data and qualitative observations. Include at least one scientific drawing.
- Calculations: Show a sample calculation for the percentage of NaHCO3 using the data you collected. Complete calculations for the class data set by making a copy of the spreadsheet and including a note in the lab notebook for where this data can be found.
Typed Final Report – Submit in Canvas
- Calculations Spreadsheets: Make a copy of the class data spreadsheet and complete data analysis. Determine the average percentage of NaHCO3 in the mixture and standard deviation. Include a link in your typed final report to the spreadsheet.
- Findings and Conclusions: Discuss what you did and why. Describe major findings and make claims supported by evidence: the percentage of NaHCO3 based on your data and the average percentage based on the class data set.
- Post Lab Question: In what real-world situations might chemists need to determine the percent composition of a mixture? Provide one specific example.
- Error Analysis: Identify at least two possible sources of error. For each, explain whether it would cause your calculated percent NaHCO₃ to be too high, too low, or unpredictable. Errors should be clearly described along with how they impacted the calculations in the analysis. Include suggestions to reduce errors if the lab were repeated.
Return to AP Chemistry Labs.
