Challenge
Your goal is to investigate the relationship between potential energy and the distance between atoms, based on factors that influence the attraction and repulsion between atoms.
Background Questions
- Why does the potential energy of a system decrease when two atoms form a stable bond?
- What happens to the potential energy of a system when repulsive forces dominate as atoms get very close?
- Why does breaking bonds always require energy input, while forming bonds always releases energy?
- Using the bar graph model, explain why the overall potential energy of the system decreases during most chemical reactions.
- How does the balance of attractive and repulsive forces determine whether a bond is stable or unstable?
Background
Atoms interact through a balance of attractive and repulsive forces. At large distances, there is little to no interaction. As atoms move closer together, the electrons of one atom are attracted to the positively charged nucleus of the other, lowering the potential energy of the system. If they get too close, however, repulsion between the nuclei and repulsion dominates, raising the potential energy sharply.
This balance of forces creates a potential energy curve (see Figure 1). The lowest point of the curve represents the bond length, the optimal distance between nuclei where attraction and repulsion balance. At this distance, the potential energy of the system is at a minimum. Because systems naturally move toward lower potential energy, bond formation is a stabilizing process. The depth of the potential well corresponds to bond strength—a deeper well indicates a stronger bond that requires more energy to break.
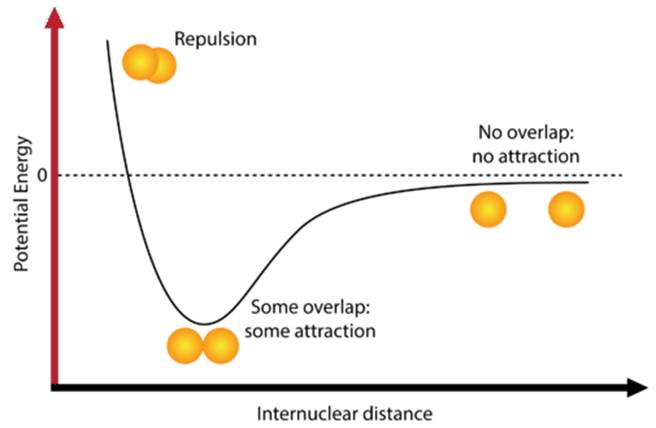
Figure 1: Potential Energy Curve
Chemical reactions involve both breaking old bonds and forming new ones. Breaking bonds requires an input of energy (endothermic step) because atoms must be pulled away from their lowest-energy arrangement. Forming bonds releases energy (exothermic step) because the system moves to a lower potential energy state. The net energy change of a reaction depends on the balance of these processes.
The bar graph below (Figure 2) illustrates the change in energy distribution during the combustion of hydrogen and oxygen gas to form water vapor. Initially, atoms bonded together have low potential energy, but some stored energy in the system. When bonds break, the potential energy increases because the atoms are no longer in their stable arrangement. When new bonds form, the potential energy drops again, and the difference in energy is released as heat or light. This is a combustion reaction that releases a significant amount of energy, so when the products are formed, most of the system’s energy exists as light and heat. Notice that the total energy of the system remains constant (energy is conserved).
Also, note that the reaction could be run in reverse, but a greater amount of energy must be absorbed and converted into potential energy to break the bonds in H2O to form H2 and O2.

Figure 2: Energy Distribution During a Chemical Reaction
This explains why reactions tend to lower the overall potential energy of the system: products usually contain bonds that are stronger and more stable than those in the reactants. The released energy often drives other processes, such as providing heat in combustion or powering biological reactions.
Using the PhET Atomic Interactions simulation, you will model these processes at the atomic level. By adjusting the distance between atoms, you will visualize the interplay of attractive and repulsive forces, observe potential energy changes, and connect these patterns to real chemical bonding and reaction energetics.
Procedures
- Access the PhET Atomic Interactions simulation (phet.colorado.edu/en/simulations/atomic-interactions).
- Provide three screenshots, one showing two atoms with a net attraction to each other, another showing two atoms with a net repulsion to each other, and another showing attractive and repulsive forces in balance.
- Print two copies of each screenshot (one for the original page and one for the copy of the lab notebook). Comment on each screenshot to explain how the attractive and repulsive forces impact the behavior of each atom pair.
- Look at the potential energy graph, and note the σ and ε. What do these values represent? Explain the basis of your answer.
- The three screenshots count as your three drawings.
- Include general observations from the simulation.
- For sources of error, focus on the assumptions, limitations, and reliability of the simulation.
Post Lab Instructions
Lab Notebook – Complete in Notebook
- Ensure all sections from the Lab Report Guidelines and Expectations are included in the lab notebook. Label each section clearly.
- Lab Notebook Set Up: experiment number, name of the experiment
- Purpose or Question: Describe what the experiment is testing or exploring and why it matters in a couple of sentences.Methods: Briefly describe what you did so someone else could repeat it.
- Data and Observations: Include both quantitative data and qualitative observations. Your screenshots count as the scientific drawing.
Typed Final Report – Submit in Canvas
- Findings and Conclusions: Discuss what you did and why. Describe major findings and make claims supported by evidence
- Post Lab Questions:
- Comment on each screenshot to explain how the attractive and repulsive forces impact the behavior of each atom pair.
- Look at the potential energy graph, and note the σ and ε. What do these values represent? Explain the basis of your answer.
- Which of the following pairs shown below is the most likely to exist as a diatomic molecule, based on their potential energy graphs? Does this make sense given that neon and argon are noble gases, and tend to not form bonds? Explain.

- Error Analysis: Identify at least two limitations of the simulation or assumptions made in the model. For each, explain how this could affect the accuracy of your observations or conclusions. Be specific about whether the limitation would likely lead to an overestimation, underestimation, or oversimplification of real atomic interactions.
Return to AP Chemistry Labs
